Oxidation Number of K
Web The O 2-TPO spectra showed that the oxidation of Ru started at a much higher temperature for the RuTiO 2 210 C than for the RuC 155 C Supplementary Fig. The oxidation number of oxygen in a compound is -2 except in peroxides when it is -1.

How To Find The Oxidation Number For Cr In K2cr2o7 Potassium Dichromate Youtube
The oxidation number is the hypothetical charge of an atom in a molecule or ion and it is a measure of its apparent capacity to gain or lose electrons within that species.

. The oxidation number of fluorine is always 1. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it. Chlorine bromine and iodine usually have an oxidation number of 1 unless they.
The sum of oxidation numbers in a neutral compound is 0. Web The oxidation number of hydrogen in a compound is 1 except in metal hydrides such as NaH when it is -1. Web Place Markers let you know where in the sequence a particular random number falls by marking it with a small number immediately to the left.
BYJUS online oxidation number calculator tool makes the calculation faster and it displays the oxidation number in a fraction of seconds. 14a indicating a much lower. Web Valency is different from the oxidation number and it has NO SIGN.
B The normalized XANES spectra at the Pt L 3 edge of sample A sample B PtO 2 and Pt foil. The sum of the oxidation numbers in a monatomic ion is equal to the overall charge of that ion. Oxidation occurs when the oxidation number of an atom becomes larger.
The data show a decreasing trend in the white. 5 3 42 18 20 This is the default layout Research Randomizer uses. With Place Markers Off your results will look something like this.
The oxidation number of an atom in elemental form is 0. Oxidation and reduction are therefore best defined as follows. Web Oxidation Number Calculator is a free online tool that displays the oxidation number of the given chemical compound.
Web In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. Reduction occurs when the oxidation number of an atom becomes smaller. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it.
While fully ionic bonds are not found in nature many bonds. Web Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change. For any neutral compound the sum of the oxidation numbers must equal 0.
Web The oxidation state of carbon increases from 2 to 4 while the oxidation state of the hydrogen decreases from 1 to 0. Web a The k 3-weighted Fourier transform spectra from EXAFS. Thus the valency of nitrogen is 3 whereas it can have oxidation numbers from -3 to 5.
Web The oxidation number of any atom in its elemental form is 0. 2 17 23 42 50 Set 2.
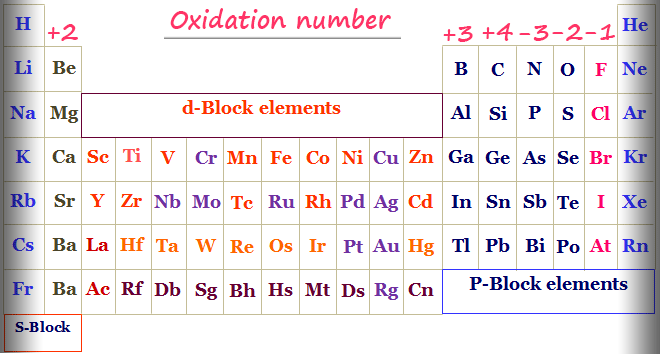
How To Calculate The Oxidation Number By Chemistry Topics Inorganic Chemistry Topics Medium

Oxidation Number State Definition Rules How To Find And Examples

How To Find The Oxidation Number For Mn In Kmno4 Potassium Permanganate Youtube

0 Response to "Oxidation Number of K"
Post a Comment